Magnesium Di-Potassium- EDTA
INTROUDUCTION:
Chelation has been done the same way for the last 50 years, namely by intravenous drip and with the ingredient Di-Sodium EDTA. In trying to improve upon chelation we looked at both the method of delivery and the ingredient being used.
The idea of oral chelation was not feasible since the absorption of EDTA given orally is about five percent due to denaturing by stomach acids. Giving larger doses of EDTA orally to make up for this lack of absorption would not work since even in small amounts Di-Sodium EDTA causes ulcerations and hemorrhaging in the colon and digestive tract. (When Di-Sodium EDTA is administered by I.V. anesthetics must be injected into the EDTA solution). The alternative to using large oral doses of Calcium Di-Sodium EDTA orally (which does not burn tissue) is not feasible since Calcium Di-Sodium EDTA cannot effectively remove calcium from the body (a vital component of chelation therapy). This is due to the fact that the calcium is already attached to the EDTA molecule and oral EDTA is not well absorbed.
It was reasoned that if a form of EDTA could be developed which would not burn tissue and did not contain calcium, suppository chelation would be possible since (unlike the stomach with a very acidic ph) the colon has a fairly neutral ph (~ 6.5 pH).
To this end, Magnesium Di-Potassium EDTA suppositories have been developed. Unlike Di-Sodium EDTA, Magnesium Di-Potassium EDTA does not burn healthy tissue. Also, due to its higher solubility (potassium increases the solubility level) and low molecular weight (408.74) it easily passes through the colonic mucosa into the general and hepatic circulation.
Dr. Halstead, considered by many to be the father of modern chelation, had this to say about suppository EDTA chelation:
“I have been involved in the development of EDTA suppositories since the idea was first conceived 7 years ago. The suppository delivery system was developed because it meets a special need. The primary purpose was to produce a drug delivery system that was painless and effective for children and for adults that found it difficult to take chelation because of time restraints. Research studies showed that the uptake of EDTA was effective by the colonic route.”
There are many other benefits associated with Magnesium Di-Potassium EDTA, not caused by the EDTA, but related to the minerals themselves. Although the amount of magnesium and potassium in 365 milligrams of Magnesium Di-Potassium, EDTA is small (21.9 mg magnesium and 69.35 mg potassium) when brought into the body with EDTA as a carrier, the effect on the autonomic nervous system can be quite profound.
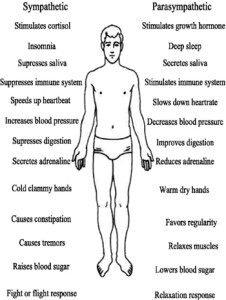
The Sympathetic and Parasympathetic Nervous Systems
The autonomic nervous system (ANS) is divided into two parts; the sympathetic system (SNS) and the parasympathetic system (PNS). The SNS creates what is commonly known as the “fight or flight response”. In the event of a perceived emergency, the body will suppress all non-critical physiological systems and push the blood into the muscles for quick action. Non-critical systems include digestion,reproduction, gestation, milk production, immune function, higher brain functions, growth and repair processes, as well as sleep. That is not to say that any SNS response is bad. For instance, it’s the SNS that keeps a person from passing out due to the effects of gravity upon their blood when they stand up quickly. The point is that continual activation of the SNS is very detrimental to our health.
The PNS is the counterpoint to the SNS. It generates what might be termed the “relaxation response”. When activated it helps suppress the SNS. When the PNS is activated, the body digests well, reproduction, gestation, and milk production are supported, the immune system is active, our brains are happy and functional, injured tissue is repaired, new tissue is formed, and we get a good night’s sleep.
Thus, it makes sense to keep the body in a slightly PNS dominant state whenever possible. This can be accomplished very easily with Magnesium Di-Potassium EDTA.
To understand the effect that Magnesium Di-Potassium EDTA can have on the nervous system, we need to review the effect of minerals on the autonomic nervous system (ANS). There are four minerals that play key roles in the balance of the autonomic nervous system namely; magnesium, potassium, sodium and calcium. They work as follows:
|
Sympathetic System |
Para Sympathetic System |
|
| Magnesium |
Blocking Action |
|
| Potassium |
Stimulating Action |
|
| Sodium |
Stimulating Action |
|
| Calcium |
Blocking Action |
Magnesium Di-Potassium EDTA can have a very powerful calming effect on the nervous system by both blocking the stress response as well as stimulating the relaxation response. Many clients report feelings of profound relaxation within 5 to 20 minutes of taking Magnesium Di-Potassium EDTA precisely because of how it affects the (ANS). On the other hand, Di-Sodium EDTA and Calcium Di-Sodium EDTA can do the exact opposite. They turn off the relaxation response and stimulate the fight or flight response. This is most easily observed by the increased pulse rate these forms of EDTA can cause.
The Removal of Calcium
As we age calcium accumulates in the soft tissues of the body. When deposited in dead tissue it is called dystrophic calcium (like atherosclerotic plaques). When deposited in living tissue it is called metastatic calcium (like arteriosclerosis). When calcium gets into a cell, the cell turns on … whatever “on” is for that cell. If it is a muscle cell that the calcium enters the muscle contracts. If the calcium stays there the muscle stays contracted. Those familiar knots in upper backs and necks are just such calcified muscles that are forever in the “on” or contracted position. The pathological version of this is fibromyalgia. In that case there are innumerable knotted muscles in clients’ bodies. The extreme example of this is rigor mortis in which all the muscles of the body flood with calcium and contract. As we age we accumulate more and more dystrophic and metastatic calcium becoming stiffer and stiffer.
One of the best ways to remove dystrophic and metastatic calcium is through chelation. EDTA removes calcium in the same way it removes other toxic metals. There are four binding sites on each EDTA molecule. These sites are capable of grabbing on to one or one-half of another metallic atom. Since calcium is a metallic element EDTA can readily form a chelated complex with it.
EDTA makes a reversible bond with metallic elements and can let go of one metal in exchange for another. To understand how this works look at the chart on the next page. As you move up and to the left of the chart, the bond between EDTA and the metals becomes stronger. EDTA can let go of a metal for any other metal to the left of the chart. Let’s start with Magnesium EDTA and see what happens. Notice that magnesium (Mg) is to the right of calcium (Ca) on the chart. This shows that EDTA has a greater affinity for and makes a stronger bond with calcium than it does with magnesium. If Magnesium EDTA meets calcium in the bloodstream, the EDTA will drop the magnesium and attach to the calcium instead. Where does this calcium come from? It comes from the bloodstream as well as from the metastatic and dystrophic calcium in the body. We now have a Calcium EDTA floating in the bloodstream. Look at the chart again and you will see that to the left of calcium is lead (Pb). Calcium EDTA will drop its calcium in exchange for the lead. You now have free-floating calcium that can be utilized to build bone and teeth and lead EDTA, which is a very stable complex. In a few hours, the lead EDTA will be removed from the body through the urine and stool. You must be sure that the EDTA you use has not already been reacted with calcium. If it has it can no longer remove metastatic or dystrophic calcium. It can only remove minerals for which it has a greater affinity (to the left of calcium on the chart). While a Calcium EDTA will do fine in the removal of lead and other toxic metals, its chemistry does not allow it to drop its calcium in exchange for another calcium, and so is limited in its therapeutic effect.
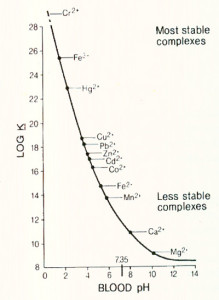 |
Since the removal of metastatic calcium is a vitally important part of chelation, make sure that you use an EDTA that only has minerals bound to it to the right of calcium on the chart. Though not listed on this chart, potassium is to the right of calcium.
The Mecury Controversy
Mercury is both the most toxic of all non-radioactive metals as well as the most ubiquitous in people. Virtually everyone is guaranteed daily exposure of this toxic metal due to the practice of putting mercury in dental fillings. Some 22 million tons of mercury are put into fillings every year. To put the toxicity of mercury in perspective were a single silver (mercury) filling to be removed from a client’s mouth and put into a ten acre lake, the EPA would have to order a fish advisory for the lake. Fishing, swimming, bathing, boating in that lake would all be off limits.To remove mercury the chelators DMPS and DMSA are often used but both have serious drawbacks.
DMPS has been shown to cause the following:
· Auto-reactive T-cells· Increases in T-suppressor cells· Liver toxicity· Kidney damage
· Stomach ulcers
DMSA has been shown to cause the following side effects: · Bone marrow suppression
· Increases in tumor necrosis factor· Liver toxicity· Nausea· Diarrhea· Anorexia
· Fatigue
· Flatulence
EDTA can chelate mercury without any of the side effects associated with DMPS and DMSA, just not as quickly. As you can see on the chart from the previous page, EDTA makes a very stable bond with mercury. It was previously thought that EDTA could not remove mercury in vivo. This was believed because it had never been seen to show up in the urine on a provocative EDTA challenge test. This is only partially correct. Under most circumstances mercury bound EDTA, will not come out of the urine. It will, however, come out of the stool (no one thought to look for it there). You can get mercury bound EDTA to come out in the urine by giving a large amount of EDTA over a short period of time but, this is not recommended. The kidneys are very sensitive to mercury. If the body can rid itself of mercury through the stool so much the safer. Since mercury is normally detoxified via the colonic route, EDTA suppositories may prove to be more effective than I.V. EDTA chelation for this purpose given that the highest concentration of EDTA in the suppositories is being delivered right to the colonic mucosa.
The Removal of Iron
It is known that premenopausal women have a lower risk of heart attack and cancer than do men of the same age. The advantage is partially due to the monthly loss of iron in the menses. This is confirmed by the fact that postmenopausal women lose this advantage, while men and women who donate blood as little as once a year have the same reduced risk as premenopausal women.
Chelation has an affinity for iron as shown on the chart on page five. If you look on that chart, you will see that EDTA can remove two different forms of iron; both the metabolically active Fe2+ and the oxidative Fe3+ forms of iron, but that it preferentially bonds to the oxidative Fe3+ form as evidences by Fe3+ being to the left of Fe2+ on the chart. This is an important distinction since Fe3+ is quite a problem in the body.Methemoglobinemia is the clinical state in which circulating hemoglobin is present with iron in the oxidized Fe3+ state instead of the usual Fe2+ state. Fe3+ cannot carry oxygen. Fe 3+ also moves the oxyhemoglobin dissociation curve to the left, thus what little oxygen is present in other hemoglobin molecules has a harder time being released into the tissues. The lack of oxygen is obvious from the chocolate brown appearance (see chart below) of methemoglobineminic blood.
In health a small amount of oxidized Fe3+ iron is formed by auto-oxidation of the circulating red blood cells. Fe2+ is oxidized to Fe3+ at the rate of about three percent per day. This iron is reduced back to Fe2+ by the enzyme NADH-cytochrome b5 reductase and reduced glutathione.
Perhaps the gradual decrease of tissue oxygen seen as we age (see chart below) is due to the downgrading of these antioxidant systems. While enzyme NADH-cytochrome b5 reductase may be upregulated with niacin, this enzyme accounts for perhaps only five percent of the reduction of Fe3+ to Fe2+. Glutathione reduces Fe3+ to Fe2+ non-enzymatically but it is a difficult antioxidant to raise in the body. Perhaps our best defense against Fe3+ for now is the use of EDTA.
Since local anesthetics cause the oxidation of Fe2+ to Fe3, and I.V. chelation is usually given with procaine to numb the burning sensation caused by Di-Sodium EDTA, we see yet another advantage of suppository chelation with Magnesium Di-Potassium EDTA over I.V. chelation. Magnesium Di-Potassium doesn’t cause the conversion of Fe2+ to Fe3 (thus lowering oxygen carrying and delivery capacity) as no anesthetics are required.
Toxic Metals and Chelation
We are continuously exposed to toxic metals in our environment. We use the term toxic metal rather than heavy metal for the following reason: A heavy metal is defined as a metal with a specific gravity five or more times that of water. Therefore; zinc, iron, manganese and chromium (all necessary metals) are heavy metals. Aluminum on the other hand has a specific gravity less than five times that of water and so is a light metal. Clearly the “weight” of the metal does not determine its toxicity. To measure metal toxicity a baseline test of the urine must be taken. It should be measured from the first urine of the day as well as any urine passed during the sleeping hours. This will show the amount of toxic metals the body is currently removing without assistance. Then, a second test, called a challenge or a provocation test should be done. To do this test the client takes an EDTA suppository in the evening. All urine passed, after the insertion of this suppository (up to and including the first urine of the next day) should be collected. This will show the levels of toxic metals coming out as a result of the EDTA. The second test can be repeated every few months depending on the severity of the readings. Keep in mind that mercury will not show up in the urine unless huge doses of EDTA are taken, otherwise they will be in the stool for which a fecal metal test would be required.
Here is a humorous look at a serious situation. This “recipe” is the average amount of each toxic metal a person will have taken into their bodies in their lifetime.
•1,460 grams of aluminum*
•1 tsp arsenic
•1 tsp lead
•1/3 tsp mercury
•1 tsp nickel
•Sprinkling of cadmium (300 mg)
•1 pinch of uranium (80 mcg) *A 12-inch square of aluminum foil is about five grams. A great visual aid to clients is to have a crumpled up ball of aluminum foil sitting on your desk. Hand it to them and explain that they will ingest the equivalent of 292 of these balls in their lifetime.